Heats of reaction and hess’s law lab – In the realm of chemistry, the concepts of heats of reaction and Hess’s Law hold immense significance. This engaging lab-based exploration delves into these fundamental principles, providing a comprehensive understanding of their implications and practical applications. By embarking on this journey, we unveil the secrets behind exothermic and endothermic reactions, unravel the intricacies of Hess’s Law, and witness the power of these concepts in shaping various fields of science and engineering.
Introduction

Chemical reactions involve the making and breaking of chemical bonds, resulting in energy changes. These energy changes are classified as either exothermic or endothermic.
Exothermic Reactions
In exothermic reactions, energy is released in the form of heat. This occurs when the bonds formed in the products are stronger than the bonds broken in the reactants, resulting in a net release of energy. A common example of an exothermic reaction is the combustion of fuels, such as burning propane in a gas stove.
Endothermic Reactions
In contrast, endothermic reactions require energy input to proceed. This occurs when the bonds formed in the products are weaker than the bonds broken in the reactants, resulting in a net absorption of energy. A common example of an endothermic reaction is the electrolysis of water, which requires an external energy source to separate water molecules into hydrogen and oxygen.
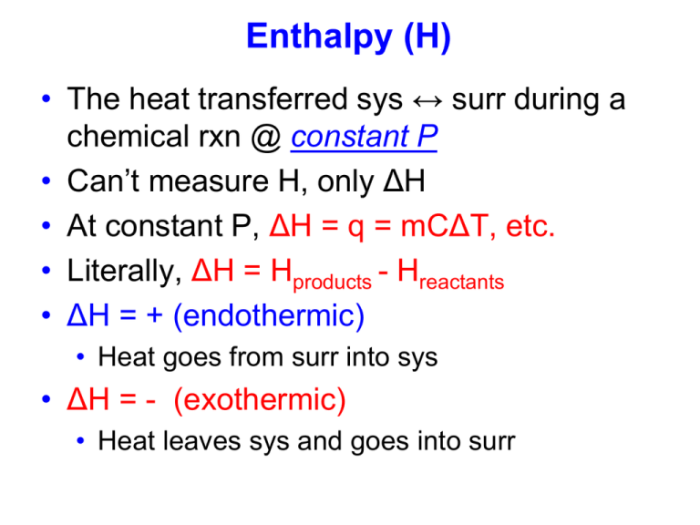
Heat of Reaction
The heat of reaction is a quantitative measure of the energy change that occurs during a chemical reaction. It is defined as the amount of heat released or absorbed per mole of reactant or product. The heat of reaction is typically expressed in units of kilojoules per mole (kJ/mol).A
positive heat of reaction indicates an exothermic reaction, while a negative heat of reaction indicates an endothermic reaction. The magnitude of the heat of reaction provides information about the strength of the bonds involved in the reaction and the overall energy change that occurs.
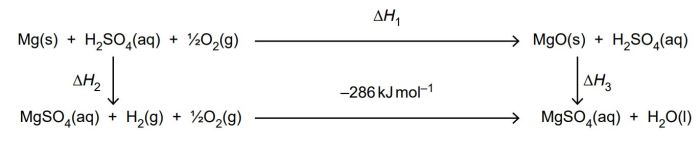
Hess’s Law
Hess’s Law states that the overall enthalpy change for a chemical reaction is independent of the pathway taken. This means that the heat of reaction for a complex reaction can be determined by adding up the heats of reaction for the individual steps in the reaction pathway.
Hess’s Law can be used to predict the heat of reaction for complex reactions. For example, the heat of reaction for the combustion of methane can be determined by adding up the heats of reaction for the following steps:
- CH4(g) + 2O 2(g) → CO 2(g) + 2H 2O(g) ΔH =
890 kJ/mol
- CO 2(g) + H 2O(g) → H 2CO 3(aq) ΔH =
110 kJ/mol
- H 2CO 3(aq) → H 2O(l) + CO 2(g) ΔH = +41 kJ/mol
The overall heat of reaction for the combustion of methane is the sum of the heats of reaction for the three steps, which is -890 kJ/mol + (-110 kJ/mol) + (+41 kJ/mol) = -959 kJ/mol.
Lab Experiment: Heats Of Reaction And Hess’s Law Lab
To determine the heat of reaction for a given reaction, an experiment can be designed using the following steps:
The experiment involves measuring the temperature change of a known mass of water when the reaction occurs. The heat of reaction is then calculated using the specific heat capacity of water and the mass of water used.
Materials and Equipment
- Reaction vessel (e.g., calorimeter, beaker)
- Thermometer
- Balance
- Water
- Reactants
- Insulating material (e.g., Styrofoam)
Experimental Procedure
- Calibrate the thermometer by immersing it in a known temperature bath.
- Measure and record the mass of the reaction vessel and water.
- Add the reactants to the reaction vessel and stir to dissolve.
- Insulate the reaction vessel to minimize heat loss to the surroundings.
- Record the initial temperature of the water.
- Initiate the reaction and stir continuously.
- Record the highest temperature reached during the reaction.
- Calculate the heat of reaction using the formula:
Heat of reaction = (mass of water) x (specific heat capacity of water) x (change in temperature)
Data Analysis

The heat of reaction can be calculated from the experimental data by measuring the change in temperature of the calorimeter and the mass of the reactants and products. The specific heat capacity of the calorimeter and the reactants and products must also be known.
The heat of reaction is given by the equation:
“`Q = m
- C
- ΔT
“`where:* Q is the heat of reaction in joules
- m is the mass of the reactants and products in grams
- C is the specific heat capacity of the calorimeter and the reactants and products in J/g°C
- ΔT is the change in temperature in °C
Sources of Error
There are several sources of error in this experiment, including:
- Heat loss to the surroundings
- Inaccurate measurement of the temperature change
- Inaccurate measurement of the mass of the reactants and products
- Incomplete reaction
These sources of error can be minimized by using a well-insulated calorimeter, carefully measuring the temperature change, accurately measuring the mass of the reactants and products, and ensuring that the reaction goes to completion.
Analysis of Results
The results of the experiment can be compared to theoretical predictions using the enthalpy of formation of the reactants and products. The enthalpy of formation is a measure of the heat released or absorbed when a compound is formed from its elements.
The enthalpy of reaction can be calculated using the following equation:
“`ΔH = ΣΔH°f(products)
ΣΔH°f(reactants)
“`where:* ΔH is the enthalpy of reaction in kJ/mol
ΔH°f is the enthalpy of formation of the products and reactants in kJ/mol
The enthalpy of reaction can be used to predict the heat of reaction. If the enthalpy of reaction is positive, the reaction is endothermic and heat is absorbed from the surroundings. If the enthalpy of reaction is negative, the reaction is exothermic and heat is released to the surroundings.
The results of the experiment can be used to verify the theoretical predictions. If the experimental heat of reaction is close to the theoretical heat of reaction, then the theoretical predictions are correct. If the experimental heat of reaction is significantly different from the theoretical heat of reaction, then the theoretical predictions may need to be revised.
Applications
Understanding heats of reaction and Hess’s Law has numerous practical applications across various fields, including chemistry, engineering, and medicine. These concepts play a crucial role in optimizing chemical processes, designing new materials, and predicting the behavior of chemical systems.
Chemistry, Heats of reaction and hess’s law lab
In chemistry, heats of reaction provide valuable insights into the energetics of chemical reactions. By measuring the heat released or absorbed during a reaction, chemists can determine whether the reaction is exothermic (releases heat) or endothermic (absorbs heat). This information is essential for understanding reaction mechanisms, predicting reaction rates, and designing efficient chemical processes.For
example, in the Haber process, which produces ammonia for fertilizer production, the heat of reaction is used to optimize the reaction conditions and maximize ammonia yield. By manipulating the temperature and pressure of the reaction, engineers can control the exothermic nature of the process and improve its efficiency.
Engineering
In engineering, heats of reaction are used in the design and optimization of various industrial processes. For instance, in the production of cement, the heat of reaction during the formation of calcium silicate minerals is used to control the temperature of the kiln and ensure the proper setting of the cement.Additionally,
heats of reaction are crucial in the design of heat exchangers, where the heat released or absorbed by a chemical reaction is used to transfer heat between two fluids. By understanding the heats of reaction involved, engineers can optimize the efficiency of heat transfer processes.
Medicine
In medicine, heats of reaction are used to study the energetics of biological processes. For example, the heat released during the hydrolysis of ATP (adenosine triphosphate) provides energy for many cellular processes. By understanding the heats of reaction involved in metabolic pathways, researchers can gain insights into the efficiency and regulation of these processes.Moreover,
heats of reaction are used in the design of drugs and drug delivery systems. By manipulating the heats of reaction, scientists can control the solubility, bioavailability, and efficacy of drugs, leading to improved patient outcomes.
FAQ Section
What is the difference between exothermic and endothermic reactions?
Exothermic reactions release heat into the surroundings, while endothermic reactions absorb heat from the surroundings.
How can Hess’s Law be used to predict the heat of reaction?
Hess’s Law states that the enthalpy change for a reaction is equal to the sum of the enthalpy changes for the individual steps in the reaction pathway.
What are some practical applications of understanding heats of reaction?
Understanding heats of reaction is essential for designing chemical processes, optimizing fuel efficiency, and predicting the stability of materials.